
Why macrophages matter in the fight against cystic fibrosis
Lung macrophages play a significant role in the chronic inflammation associated with cystic fibrosis
Antibody-drug conjugates (ADCs) have revolutionised cancer therapy, offering targeted and effective treatment options. By leveraging monoclonal antibodies (mAbs) to deliver cytotoxic agents directly to cancer cells, ADCs aim to minimise damage to healthy tissues, thereby reducing the adverse side effects typically associated with conventional chemotherapy. This is achieved by utilising specific antigens on cancer cell surfaces, allowing the ADC to bind selectively to these cancerous cells.
Despite their promise, a significant proportion of ADCs fail during development, with toxicity being a major hurdle1 . These failures highlight the need for advanced approaches to designing ADCs that can balance efficacy with safety.
A significant hurdle in ADCs development is the issue of off-target toxicity. This occurs when ADCs interact with healthy cells expressing similar antigens or due to the premature release of the toxic payload. Such toxicities can limit the therapeutic window of these promising drugs.
For example, ADCs are the leading cause of interstitial lung disease (ILD)2. ILD can involve direct cytotoxicity to parenchyma of the lung, alterations to health and permeability of endothelial cells, inflammation (alveolitis), and accumulation of immune cells that together, results in fibrosis and impaired lung function3. The ability to accurately predict and assess these toxicities early in the drug development process is essential for moving ADCs through the pipeline safely and efficiently.
Understanding alveolar macrophage function is crucial for assessing off-target ADC effects. These immune cells play a central role in ADC uptake, processing, and potential payload release, impacting surrounding lung tissue. Prior research indicates that ADC internalisation by macrophages can contribute to interstitial lung disease by increasing local cytotoxicity and inflammation4. Current ADC toxicity screening models lack immune cells, limiting their predictive accuracy for lung injury.
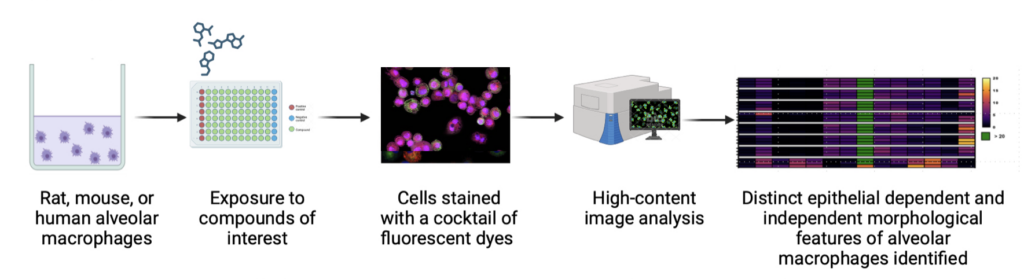
ImmuONE’s morph_ONETM assay uses alveolar macrophages to detect these off-target effects early in the development process.
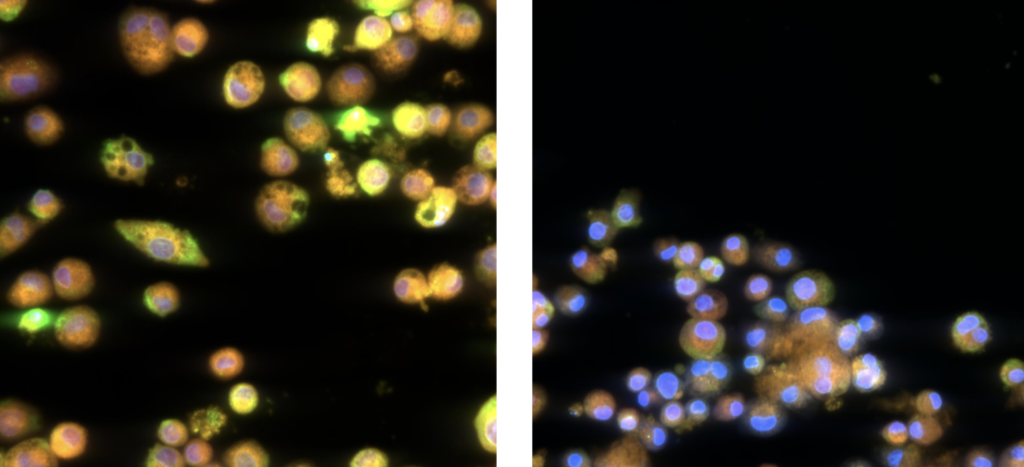
For example, in studies involving Trastuzumab deruxtecan (T-DXd), the morph_ONETM assay successfully identified that at high concentrations, adaptive macrophage morphology changes are seen rather than cytotoxic effects. Such findings highlight the assay’s capability to detect nuanced cellular responses that may not be immediately apparent with traditional toxicity assays. This focus on respiratory effects is vital for ensuring that ADCs develop with a more comprehensive understanding of their safety profiles.
ImmuONE’s morph_ONETM high-content imaging assay analyses alveolar macrophage morphology to provide insights into biological pathways, including particle overload, inflammation, phospholipidosis and apoptosis. Using advanced imaging, it captures detailed cellular images, assessing cell morphology and health indicators like nuclear integrity, cytoplasm condition, mitochondrial activity, and membrane permeability.
Implementing the morph_ONETM assay early in the ADC development process offers several benefits. By identifying potential off-target toxicities before progressing to animal studies, developers can refine candidate selection, prioritise safer compounds, and optimise dosing strategies. A proactive approach at this stage not only enhances the safety and efficacy of ADCs but also increases the likelihood of successful clinical trials.
Early detection of potential toxicities can significantly reduce development costs and timelines. By eliminating less promising candidates early, resources can be focused on compounds with a higher likelihood of success. This efficiency is crucial in the competitive pharmaceutical industry, where time-to-market can be a critical factor in a drug’s commercial success.
The future of ADC development looks promising, with technologies like morph_ONETM assay paving the way for safer and more effective therapies. As the understanding of ADC mechanisms and potential toxicities evolves, so too will the methods for assessing and mitigating these risks. The morph_ONETM assay exemplifies the potential of innovative in vitro systems to revolutionise the drug development process, providing more accurate and relevant data over traditional methods.
The ongoing development of predictive toxicity assays promises to enhance the safety profiles of ADCs, ultimately improving patient outcomes and broadening their therapeutic applications. By reducing the risks of off-target effects, these innovations will facilitate the development of ADCs that are not only more effective but also safer for patients. As research progresses, the insights gained from these tools will undoubtedly contribute to the ongoing evolution of precision medicine, offering new hope in the fight against diseases.
1 https://pubmed.ncbi.nlm.nih.gov/36765668/
2 https://pubmed.ncbi.nlm.nih.gov/39660786/
3 https://pubmed.ncbi.nlm.nih.gov/37185443/
4 https://pmc.ncbi.nlm.nih.gov/articles/PMC11791479/
5 ImmuONE’s research work on ADC’s. Download full research and case study below…

Lung macrophages play a significant role in the chronic inflammation associated with cystic fibrosis

Inhaled substances are primarily tested on rats for toxicity, but key differences between rat and human lungs suggest it’s time to look towards alternative methods.

We’ve just returned from the Society of Toxicology conference in Nashville, where we were excited to exhibit our upcoming in vitro cell culture models.
Make Every Breath Safe
Sycamore House
16 Leyden Road
Stevenage, Hertfordshire, UK
SG1 2BP
Copyright 2025 ImmuONE.
All rights reserved.