ImmuBYTES Session 3 Video Recording
Early detection of pulmonary ADC toxicity using ImmuONE’s morph_ONE™ high-content imaging assay of alveolar macrophages to improve safety and efficacy in ADC drug development.
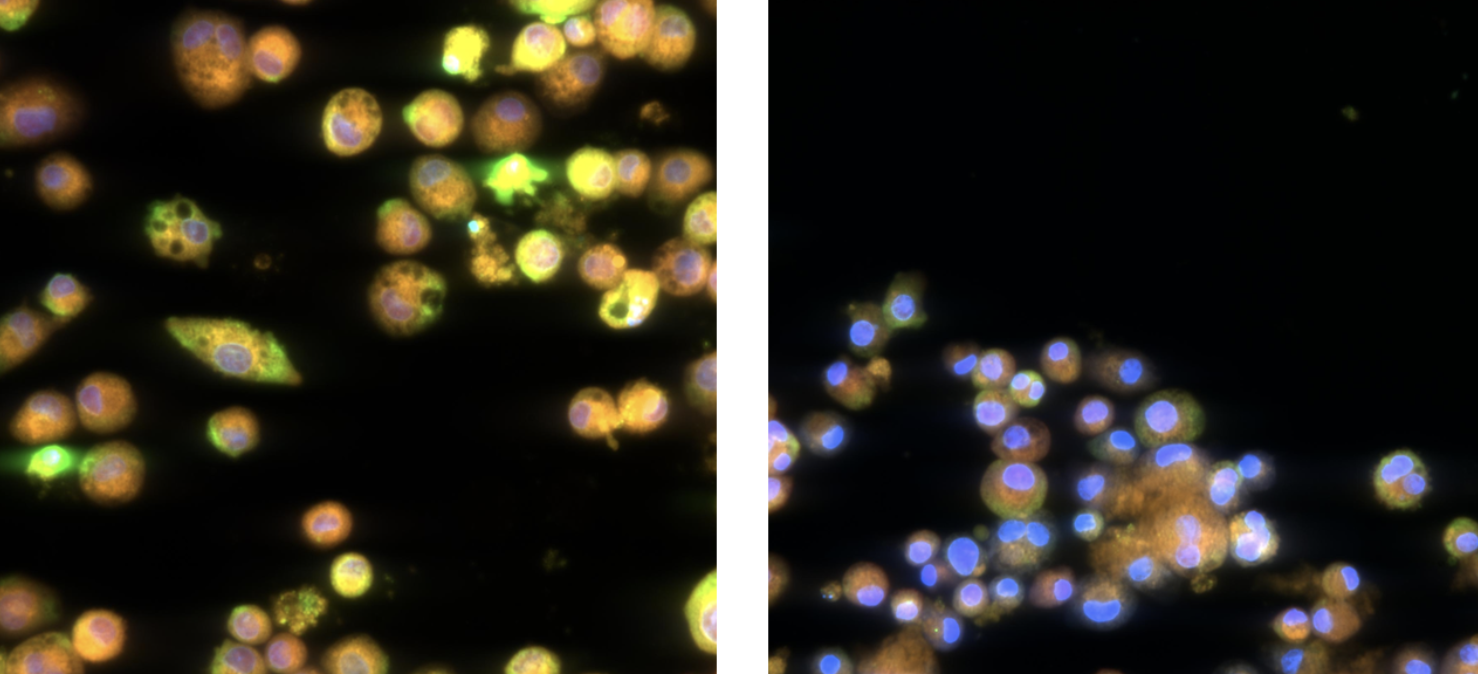
The lipid_ONE™ assay offers comprehensive in vitro lipid profiling and morphometric assessment within macrophages in response to inhaled substances.
Our multiparametric cell painting tool enables precise phenotyping, categorisation, and comparison of individual cells based on multiple characteristics. This enhances safety assessments, identifying adverse effects and minimising candidate compound failures in later drug development stages.

lipid_ONE is designed to assess changes in phospholipid and neutral lipid content within cells.
It predicts how intracellular lipid profiles alter with compound exposure to provide mechanistic-driven safety approaches for a range of adverse responses (phospholipidosis, autophagy) and initiating events in diseases (atherosclerosis, steatosis).
lipid_ONE™ is accessible as part of ImmuONE’s service packages
Alveolar macrophages are often the first cells to respond to an inhaled substance in the deep lung. Their reactions are key determinants of how inhaled substances affect the lungs.
At ImmuONE, we strive to provide relevant immunological and biochemical readouts that can be linked to mechanisms of toxicity. Most alternative cell platforms only indicate cell viability, but ImmuPHAGE™ delves much deeper, offering a unique insight into the impact of chemicals and particles on human alveoli.
In addition, ImmuONE’s scientists are highly specialised in lung alveolar interactions and can provide expert analysis of the results elicited by ImmuPHAGE™. We can benchmark results for a new compound against our reference database to put test results in context, helping our clients answer safety questions with confidence.
This versatile immune model can be applied to problems faced by a range of industries i.e., chemicals, consumer products, pharmaceuticals and many others.
ImmuPHAGE™ is optimised to assess the safety risks of different products, closely mimicking the effects observed in human lungs. It highlights lower airway immune processes, i.e., particle clearance, inflammation, cell functionality and morphological changes (i.e. foamy macrophages) promoting the advancement of ethical and streamlined evaluation techniques.

The model is composed of alveolar macrophage-like cells assembled in vitro in well plates, that closely represent the form and function of human cells. Substances are added to the well plates, and adverse responses can then be evaluated. ImmuPHAGE™ is fully validated and characterised for cell structure & viability, phagocytic activity and cytokine response. ImmuPHAGE™ is available for purchase as a product and as part of ImmuONE’s service packages.
Watch our video below to see how to use ImmuPHAGE™


Early detection of pulmonary ADC toxicity using ImmuONE’s morph_ONE™ high-content imaging assay of alveolar macrophages to improve safety and efficacy in ADC drug development.

In ImmuBYTES Session 3, “Looking Beyond Baseline Deviations for Lung and Immune Safety,” Prof. Victoria Hutter (CSO & Co-founder) highlighted how subtle changes in macrophage morphology can uncover early immune and lung effects.

Early detection of pulmonary ADC toxicity using ImmuONE’s morph_ONE™ high-content imaging assay of alveolar macrophages to improve safety and efficacy in ADC drug development.
Make Every Breath Safe
Sycamore House
16 Leyden Road
Stevenage, Hertfordshire, UK
SG1 2BP
Copyright 2025 ImmuONE.
All rights reserved.