Immune cells are reshaping inhalation toxicology by offering deeper insights into respiratory and systemic effects of tested compounds. In the second ImmuBYTES webinar, ‘Immune cells as predictive tool for toxicology’, Dr. Ewelina Hoffman highlighted the crucial role of alveolar macrophages in improving preclinical safety assessments.
Key highlights include:
1. Immune cells & alveolar macrophages
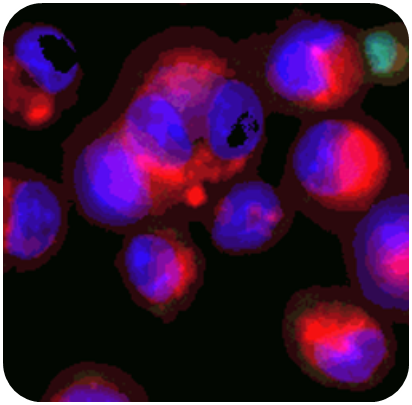
As the lungs’ first line of defence, alveolar macrophages clear foreign substances and regulate inflammation. They influence both local and systemic effects, yet conventional in vitro models often overlook them. Their foamy morphology may signal adverse or adaptive responses, emphasising the need for immune-inclusive assessments.
2. Endpoints in inhalation toxicology
Lung complexity and limitations of current testing methods make toxicity assessments challenging. ImmuONE’s four assessment packages address these gaps:
- Cell health: metabolic activity, membrane integrity, apoptosis/necrosis markers;
- Morphometric analysis: imaging and quantification of macrophage morphology;
- Inflammation: macrophage polarisation, cytokine release, oxidative stress;
- Clearance: phagocytic activity and motility assessment.
3. Profiling & baseline generation
A well established baseline of immune cells in vitro improves predictability. ImmuONE has characterised untreated rat and human macrophages, with or without epithelial cells, based on their cell health, morphology, and lipid content and applied these baselines to assess compound-induced responses.
4. Inter-species differences
Rat models, while valuable, differ significantly from humans in anatomy, metabolism, and immune response, affecting toxicity study outcomes. ImmuONE has shown rat and human alveolar macrophage differences to refine cross-species comparisons.
Looking ahead
By integrating immune cells into inhalation toxicology, we can enhance predictive accuracy and improve safety assessments.
Want to advance your research? Contact our expert team to explore how we can support your inhalation toxicity studies. See you at our next ImmuBYTES session in May.