ImmuBYTES Session 3 Video Recording
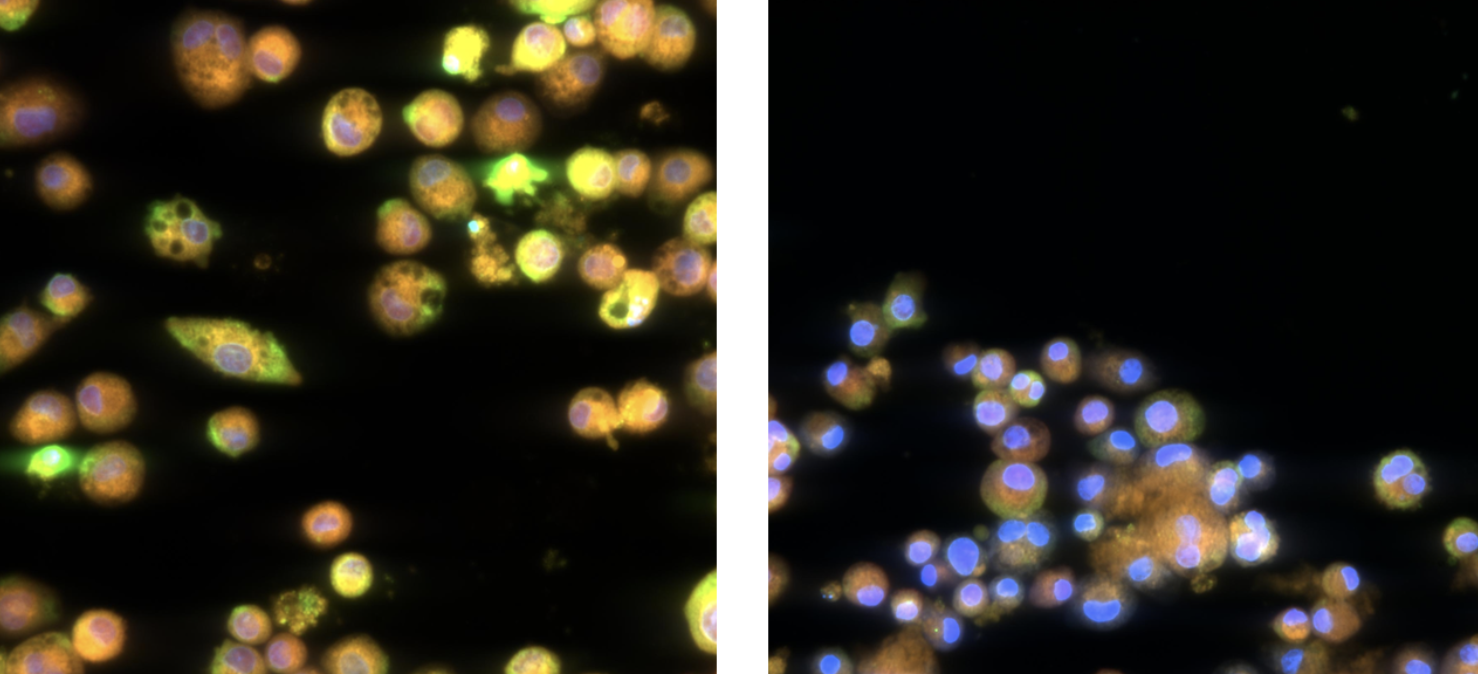
Early detection of pulmonary ADC toxicity using ImmuONE’s morph_ONE™ high-content imaging assay of alveolar macrophages to improve safety and efficacy in ADC drug development.

Following successful completion of the Phase 1 challenge awarded to ImmuONE by NC3Rs, we were announced as the winners for the Phase 2 challenge in January 2022. Sponsored by Unilever and AstraZeneca, the project aims to establish and develop in vitro OECD test guideline assays; free from animal-derived products and demonstrate their equivalence and superiority to current strategies. Once optimised, these technologies will progress to commercially ready, human relevant, animal-free bioassays with the view of gaining regulatory acceptance.

Early detection of pulmonary ADC toxicity using ImmuONE’s morph_ONE™ high-content imaging assay of alveolar macrophages to improve safety and efficacy in ADC drug development.

In ImmuBYTES Session 3, “Looking Beyond Baseline Deviations for Lung and Immune Safety,” Prof. Victoria Hutter (CSO & Co-founder) highlighted how subtle changes in macrophage morphology can uncover early immune and lung effects.

Early detection of pulmonary ADC toxicity using ImmuONE’s morph_ONE™ high-content imaging assay of alveolar macrophages to improve safety and efficacy in ADC drug development.
Make Every Breath Safe
Sycamore House
16 Leyden Road
Stevenage, Hertfordshire, UK
SG1 2BP
Copyright 2025 ImmuONE.
All rights reserved.