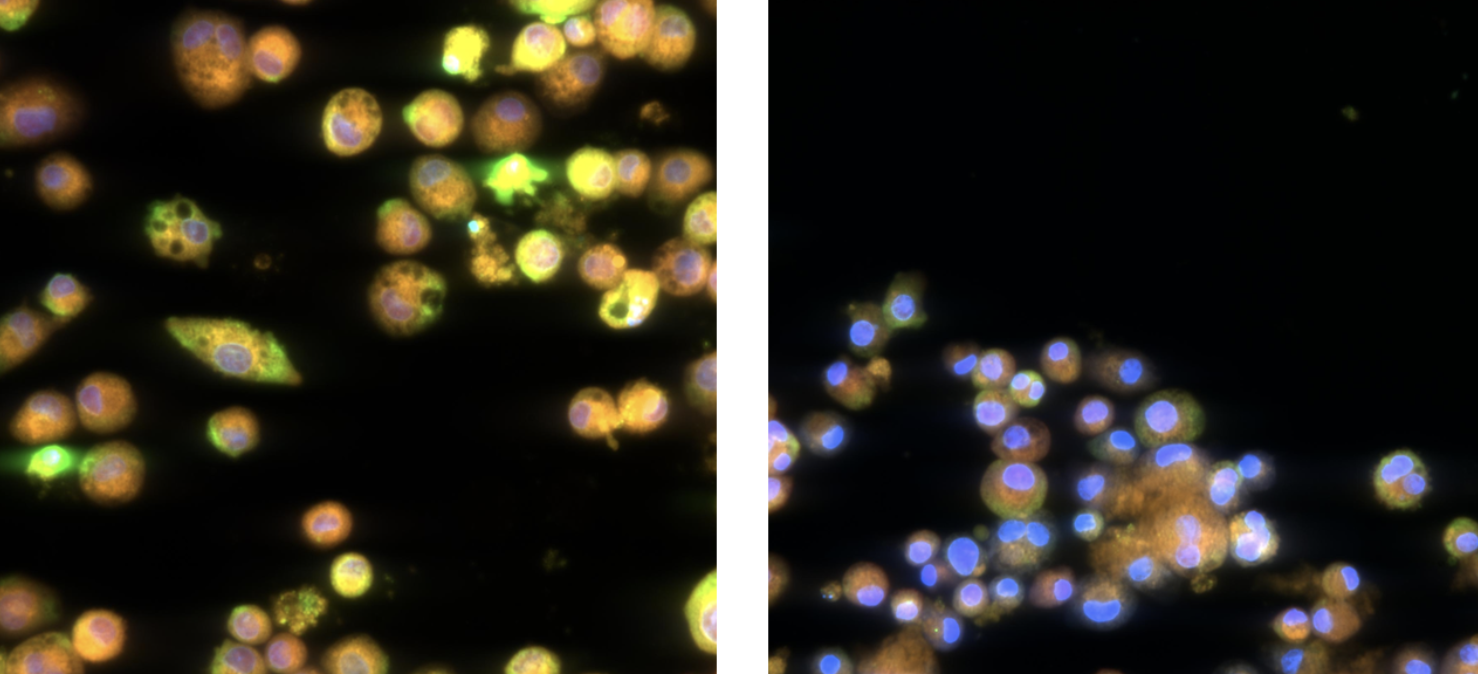
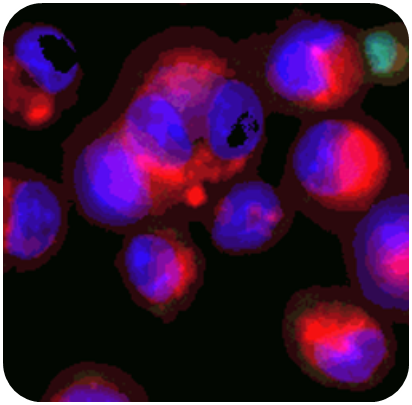
Looking beyond the baseline: new frontiers in lung and immune safety
In ImmuBYTES Session 3, “Looking Beyond Baseline Deviations for Lung and Immune Safety,” Prof. Victoria Hutter (CSO & Co-founder) highlighted how subtle changes in macrophage morphology can uncover early immune and lung effects.